4. Water Treatment To Prevent Emitter Plugging
Preventing emitter plugging is best accomplished by continuous or intermittent water treatment with organic or inorganic chemicals that are able to:
- prevent biological growths,
- prevent precipitation reactions, or
- dissolve scale deposited on the inside surfaces of tubing and emitters.
Preventative treatments greatly reduce the need for system flushing. Water treatment chemicals vary widely in stability, mode of action, corrosiveness, safety of use, dosage, and cost.
4.1. Biocides
Chlorination is the most widely used chemical irrigation water treatment to prevent biological emitter plugging. Chlorine injected into irrigation water kills microorganisms like algae and bacteria. These organisms are most commonly found in surface water, but they may also be present in ground water.
4.1.1. Chlorination
Chlorine sources may be gas, liquid, or solid:
- Chlorine gas is the most effective and economical source, but there is some resistance to its use due to high toxicity.
- Sodium hypochlorite (NaOCl) solution (household bleach) is readily available and is relatively safe to handle.
- Calcium hypochlorite (Ca(OCl)2) is a powder or pellets (swimming pool chlorine) that must be dissolved in water to form a stock solution. Calcium
- hypochlorite may cause calcium precipitation in alkaline irrigation water, so its use is not recommended.
Use Table 3 to compare the available Cl provided by other Cl sources with chlorine gas.
Source |
Available chlorine |
No. of lbs equivalent to 1 lb of chlorine gas |
Chlorine gas |
100 |
1.0 |
Calcium hypochlorite (swimming pool chlorine) |
65 |
1.5 |
Lithium hypochlorite |
36 |
2.8 |
Sodium hypochlorite (laundry bleach) |
10 |
10.0 |
Trichloroisocyanuric acid |
90 |
1.1 |
Sodium dichloroisocyanurate |
63 |
1.6 |
Potassium dichloroisocyanurate |
60 |
1.7 |
Chlorine dioxide |
4 |
25.0 |
For economy, use chlorine gas (if legal in the local area) to control bacterial slime deposits in micro-irrigation systems where continuous chlorination is needed.
- Gas chlorine is contained in steel cylinders and does not lose its strength in storage as liquid sodium hypochlorite does.
- Modern gas injectors only allow chlorine to be delivered under a vacuum. Gas is drawn from the tank by a venturi suction device driven by water flow.
- If the vacuum line breaks or if any part of the vacuum system is damaged, gas flow shuts off immediately.
Inject chlorine continuously if the irrigation water contains high algae or bacteria concentrations:
- Inject chlorine at low concentration, resulting in at least 0.5 ppm of “free” chlorine at the end of the farthest lateral line.
- No damage to plants will occur when irrigating with low chlorine water. Active chlorine is rapidly deactivated in the soil.
Inject chlorine weekly or bi-weekly to achieve about 50 ppm “free” chlorine at the end of the farthest lateral line if the water source is low in algae or bacteria.
Superchlorinate up to 500 ppm to reclaim a system that has bacterially-plugged emitters. WARNING: Super-chlorination may damage sensitive plants and irrigation system components.
For most large microirrigation systems, it takes about 20 to 30 minutes for an injected chemical to travel to the farthest emitter. Determine travel time for specific systems by:
- Injecting soap and measuring the time it takes for bubbles to appear at the farthest emitter.
- Injecting fertilizer and measuring the time it takes to observe an increase in electrical conductivity of the water at the end of the system.
Use a field test kit to measure chlorine concentration in irrigation lines:
- Analyze for free chlorine; measuring total chlorine is not as meaningful.
- Natural water has an inherent chlorine demand; use trial and error to achieve a specific free residual chlorine concentration.
- Chlorine reacts with suspended organic matter, soil particles, and other dissolved constituents.
- Hydrogen sulfide consumes about 2 ppm chlorine for each ppm of sulfide, while iron consumes about 0.7 ppm chlorine for each ppm of iron.
Skip to Top
4.1.1.2. Effects of chlorine
Chlorine has different effects, depending on concentration:
- At low concentration (1 to 50 ppm), it kills microbes and oxidizes iron.
- At higher concentration (100 to 500 ppm) it can oxidize organic matter, and can be used to disintegrate organic materials that have accumulated in emitters.
Always contact the manufacturer of your irrigation system components to verify emitter resistance to super-chlorination, since emitter parts may be made of silicon or other materials that chlorine will degrade.
Chlorine behavior in water:
- When chlorine is injected into water, free chlorine is composed of hypochlorous acid (HOCl) and hypochlorite (OCl).The reaction is shown below for chlorine gas.
| Cl2 + | H20 « | HOCl | H+ | Cl- |
| Chlorine | Water | Hypochlorous acid | Acid | Chloride |
| HOCl | « | H+ | + | OCl- |
| Hypochlorous acid | Acid | Hypochlorite | ||
The lower the water pH, the more the chlorine exists as HOCl (Fig. 1).
HOCl is about 60 times more powerful as a biocide than OCl-. For a more economical chlorine treatment, acidify alkaline water so that hypochlorous acid (HOCl) predominates.
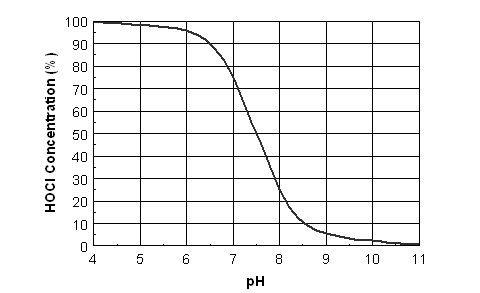
Chlorine injection example #1At a pH of 7.5, a chlorine injection rate of 10 ppm was required to maintain 1 ppm free chlorine at the end of the last lateral. If the water is acidified to pH 6.5, estimate the new liquid chlorine injection rate required to maintain the same free chloride concentration. AnswerFrom Fig. 1, the HOCl concentration at pH 7.5 is 45%, and at pH 6.5 HOCl = 90%. Therefore, 0.45 x 10 ppm = 0.90 x M M (the new injection rate) = 5 ppm. The required chlorine injection rate could be cut in half by lowering the water pH from 7.5 to 6.5. |
Skip to Top
4.1.1.3. Injecting chlorine
Use this formula to establish the starting point for liquid sodium hypochlorite injection:
I liquid chlorine = (0.006 x P x Q)/m
I = gallons of liquid sodium hypochlorite injected per hour,
p = parts per million desired,
Q = system flow rate in gpm,
m = percentage chlorine in the source (usually 5.25% or 10%).
Chlorine injection example #2Determine the liquid chlorine injection rate given these conditions: 1) the chlorine source is a 10% sodium hypochlorite solution, 2) the system flow rate is 1000 gpm, and 3) the desired concentration is 5 ppm. Answer(0.006 x 5 ppm x 1000 gal/min)/10 = 3 gph |
Use this formula when injecting chlorine gas:
I gas chlorine = (0.012 x P x Q)
I = chlorine gas injection rate (lbs/day),
P = parts per million desired,
Q = system flow rate in gpm.
Chlorine injection example #3Determine the gas chlorine injection rate given these conditions: 1) the system flow rate is 1000 gpm, and 2) the desired concentration is 5 ppm. Answer(0.012 x 5 ppm x 1000 gal/min) = 60 lbs/day |
Take these precautions when injecting chlorine:
- If injecting acid and chlorine at the same time, do so at two different injection points. Mixing acid and liquid chlorine together will produce highly toxic chlorine gas. Never store acids and chlorine together.
- Do not inject herbicides and pesticides simultaneously with chlorine because the organic chemicals may break down.
- Always add chlorine to water, not vice versa.
- Inject chlorine upstream of the filter:
- Chlorine will help keep the filter clean.
- The filter will remove precipitates caused by chlorine injection.
- Liquid chlorine deteriorates with time.
- Shield storage tanks from the sun to reduce degradation.
- Use chlorine as soon as possible after receiving it.
Skip to Top
4.1.2. Copper sulfate
Copper sulfate is occasionally used to prevent bacterial growth in micro-irrigation systems due to the toxicity of copper.
Chlorine is more effective, less expensive, and causes fewer plant toxicity and aluminum corrosion problems than copper sulfate. However, it is now included in some commercial irrigation line cleaning mixtures together with citric acid to reduce slimy bacterial growth.
Copper sulfate is commonly used for pond treatment to suppress algae. Even at relatively high concentration (around 30 ppm), copper sulfate will not be completely effective because algal spores can exclude it.
4.1.3. Chelated copper
Chelated copper acts as a bactericide similar to copper sulfate. Its advantage is that it is effective when injected at much lower rates (about 1 ppm).
It is usually not necessary to inject copper compounds for an entire irrigation cycle. Copper can be injected during the latter part of the cycle, which leaves an effective residue in the lines to prevent bacterial growth after the system is shut down.
4.2. Acidification
- Add acid to irrigation water to help prevent emitter plugging:
- Lowering the water pH can enhance the effectiveness of chlorine.
- The pH-lowering power of acid can prevent precipitation of solid compounds, particularly calcium carbonate (CaCO3).
- Citric acid has prevented iron scale formation when continuously injected at 25 ppm.
- Neutralize 80% of the bases (carbonates and bicarbonates) in the water to eliminate carbonate precipitation. (See Appendix 2 for a method to determine how much acid to inject for 80% neutralization.)
- Typical acids that can be injected to neutralize carbonates:
- Sulfuric acid
- Muriatic (hydrochloric) acid
- N-pHuric® or similar compounds (see below)
- Phosphoric acid
- Do not use phosphoric acid if there is more than 50 ppm Ca in the irrigation water because calcium phosphate will likely precipitate.
- Calcium phosphate is nearly insoluble and does not readily dissolve.
- If phosphoric acid is used at a much higher concentration for line purging (see section 6), calcium phosphate will probably not precipitate.
- N-pHuric® is a mixture of urea and sulfuric acid. It combines the benefits of adding urea nitrogen to crops with acidification while eliminating the undesirable characteristics of using sulfuric acid alone.
- Calcium phosphate is nearly insoluble and does not readily dissolve.
- Long-term acidic nitrogen fertilizers must be used with caution because:
- nitrogen should not be applied to some crops near harvest, and
- the soil pH may become too acidic with repeated applications of acid-based fertilizer.
- Use Table 4 to estimate the amount of N-pHuric® needed to neutralize excess carbonate.
| Carbonates in water | N-pHuric® 28/27 | N-pHuric® 15/49 | N-pHuric® 10/55 |
| PPM | OZ | OZ | OZ |
| 50 | 12 | 6 | 6 |
| 100 | 24 | 13 | 11 |
| 200 | 49 | 25 | 22 |
| 300 | 73 | 28 | 33 |
| 400 | 97 | 50 | 44 |
*CAUTION: Always add acid to water; do not add water to acid. Adding water to acid can cause a violent reaction, and may cause the acid to splash on the person pouring the water. Individuals working with acids should wear protective clothing and eyewear. Also, be sure that adequate safety devices are provided, including a shower and eyewash.
Skip to Top
4.3. Synthetic scale inhibitors
The most common alternative chemicals to chlorine and acids are scale inhibitors, which lessen scale formation by preventing precipitation reactions from occurring long enough for problem ions to clear the irrigation system. Inhibitors are usually synthetic polymer or polyphosphate mixtures. They are often combined with surfactants and penetrants to help break apart biological and crystalline solids attached to tubing walls and emitters. Water conditioners do not kill bacteria, but they alter the chemical environment so conditions for deposition or attachment are less favorable.
4.3.1. General information
Scale inhibitors have been used for many years in municipal water treatment and cooling tower applications.
- Some of these compounds can remove scale, while others prevent its formation by sequestering cations, particularly iron. (Sequestration keeps metal ions in suspension without removing them from the water.)
- Some industrial compounds are sold to prevent scaling and sequester iron in micro-irrigation systems.
- Inhibitors are safe and easy to handle (as opposed to acid) and are often registered for drinking water applications.
- Directly adopting industrial inhibitors for micro-irrigation may not be successful because the water chemistry may differ significantly from an industrial system.
Scale inhibitors can be costly because they are usually proprietary materials.
- They are injected into irrigation systems at rates normally less than 10 ppm to keep their use affordable.
- A water analysis can help determine the most favorable combination of chemicals in a particular mixture. Some water conditioner manufacturers will not sell a product to a customer until water sample test results are known so a scaling inhibitor can be custom blended for the particular water source.
- Initial applications of scale inhibiting chemicals to poorly maintained micro-irrigation systems might require higher rates than normally used for routine maintenance. The increased rate may be required for several applications until system cleanliness has improved to the point that only maintenance rates are required.
There is uncertainty about inhibitor selection and injected concentration required
- Based on limited experience, the best anti-plugging formulations may be a mixture of several chemicals, each with a different function.
- # A satisfactory broad-spectrum formulation would suppress slimy bacterial growth and precipitation of iron, manganese, calcium, and magnesium.
- No general recommendation for scale inhibitor use is provided in this guide because of the wide variety of commercial products available and the wide variation in irrigation water source characteristics.
- Consult the manufacturers of scale-inhibiting chemicals for recommendations on the product type that is best for your situation and the concentration at which to inject it.
- If you decide to try a scale-inhibiting chemical, evaluate its performance using the guidelines given in section 5.4.
Skip to Top
4.3.2. Polyphosphates
The most common synthetic scale inhibitors are polyphosphates:
- Polyphosphates are mixtures of various length chain molecules that have orthophosphate (PO43-) groups linked together.
- The average number of P atoms chained together ranges from 4 to 18.
There are major differences between polyphosphate compounds. Comparisons of one chemical with another are complicated and depend on water chemistry
Polyphosphates vary in their ability to trap and hold metal ions in solution. The effectiveness of a polyphosphate is determined by:
- The concentration of both the metal ion and the polyphosphate in solution.
- The relative stability of the metal ion-polyphosphate combination. For example, one polyphosphate might bind metals in the order Ca > Mg > Fe (strongest first), whereas another might bind in the order Fe > Ca > Mg.
Typically, polyphosphates cannot sequester more than 1 to 2 ppm Fe in irrigation water, and the dosage required is 2 to 5 times the Fe concentration.
Polyphosphates usually lose their potency with storage times longer than about 4 weeks.
4.3.3. Phosphonates and polyelectrolytes
Phosphonates and polyelectrolytes have also been used in municipal water industries as scale inhibitors. They differ structurally from polyphosphates.
A polymaleic anhydride anionic terpolymer is currently sold to sequester iron and manganese.
- Iron and manganese ions attach to this polymer and pass through the irrigation system rather than oxidizing and precipitating in the lines and emitters.
- Manufacturers claim these compounds provide de-scaling of certain precipitates, including calcium phosphates and calcium carbonates.
Skip to Top
4.4. Evaluating water conditioning treatments
Determine if a water conditioning treatment (injection of chlorine, acid, or a synthetic scale inhibitor) is working by monitoring the system or by installing scale-monitoring devices.
4.4.1. Monitor the system to detect plugging
Keep track of system pressure:
- A gradual pressure increase with time at constant lift and engine speed (rpm) may indicate that emitters are slowly plugging.
- Conversely, a pressure decrease observed after injection of a water conditioner would suggest that the chemical treatment is working.
Alternatively, keep track of system flow:
- A slow reduction in flow rate at the same engine speed and pressure would indicated gradual plugging.
- Increased flow rate after chemical treatment would indicate a reduction in plugging.
4.4.2. Use scale-monitoring devices to evaluate cleaning
A scale-monitoring device is a clean, non-scaled surface like a glass slide or short section of new tubing that is spliced into an irrigation lateral line.
Install several devices across the irrigation system network, from laterals close to the pump to those at the far end of the system.
Irrigate with the system as normal, and periodically check the devices for new scale deposition.
When trying a new water treatment chemical, leave untreated at least one irrigation zone that draws from the same water source as the treated zones, and install scale monitoring devices in each.
After a 4 to 6 week trial period of irrigation in treated and untreated zones, examine the scale-monitoring devices to see if less scale was deposited in the zone where the water treatment chemical was used.
Skip to Top

