Preventing emitter plugging is best accomplished by continuous or intermittent water treatment with organic or inorganic chemicals that are able to:
Preventative treatments greatly reduce the need for system flushing. Water treatment chemicals vary widely in stability, mode of action, corrosiveness, safety of use, dosage, and cost.
Chlorination is the most widely used chemical irrigation water treatment to prevent biological emitter plugging. Chlorine injected into irrigation water kills microorganisms like algae and bacteria. These organisms are most commonly found in surface water, but they may also be present in ground water.
Chlorine sources may be gas, liquid, or solid:
Use Table 3 to compare the available Cl provided by other Cl sources with chlorine gas.
Source |
Available chlorine |
No. of lbs equivalent to 1 lb of chlorine gas |
Chlorine gas |
100 |
1.0 |
Calcium hypochlorite (swimming pool chlorine) |
65 |
1.5 |
Lithium hypochlorite |
36 |
2.8 |
Sodium hypochlorite (laundry bleach) |
10 |
10.0 |
Trichloroisocyanuric acid |
90 |
1.1 |
Sodium dichloroisocyanurate |
63 |
1.6 |
Potassium dichloroisocyanurate |
60 |
1.7 |
Chlorine dioxide |
4 |
25.0 |
For economy, use chlorine gas (if legal in the local area) to control bacterial slime deposits in micro-irrigation systems where continuous chlorination is needed.
Inject chlorine continuously if the irrigation water contains high algae or bacteria concentrations:
Inject chlorine weekly or bi-weekly to achieve about 50 ppm “free” chlorine at the end of the farthest lateral line if the water source is low in algae or bacteria.
Superchlorinate up to 500 ppm to reclaim a system that has bacterially-plugged emitters. WARNING: Super-chlorination may damage sensitive plants and irrigation system components.
For most large microirrigation systems, it takes about 20 to 30 minutes for an injected chemical to travel to the farthest emitter. Determine travel time for specific systems by:
Use a field test kit to measure chlorine concentration in irrigation lines:
Chlorine has different effects, depending on concentration:
Always contact the manufacturer of your irrigation system components to verify emitter resistance to super-chlorination, since emitter parts may be made of silicon or other materials that chlorine will degrade.
Chlorine behavior in water:
| Cl2 + | H20 « | HOCl | H+ | Cl- |
| Chlorine | Water | Hypochlorous acid | Acid | Chloride |
| HOCl | « | H+ | + | OCl- |
| Hypochlorous acid | Acid | Hypochlorite | ||
The lower the water pH, the more the chlorine exists as HOCl (Fig. 1).
HOCl is about 60 times more powerful as a biocide than OCl-. For a more economical chlorine treatment, acidify alkaline water so that hypochlorous acid (HOCl) predominates.
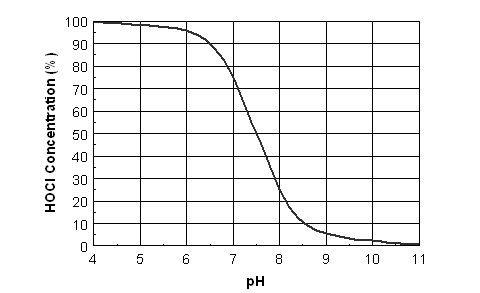
Chlorine injection example #1At a pH of 7.5, a chlorine injection rate of 10 ppm was required to maintain 1 ppm free chlorine at the end of the last lateral. If the water is acidified to pH 6.5, estimate the new liquid chlorine injection rate required to maintain the same free chloride concentration. AnswerFrom Fig. 1, the HOCl concentration at pH 7.5 is 45%, and at pH 6.5 HOCl = 90%. Therefore, 0.45 x 10 ppm = 0.90 x M M (the new injection rate) = 5 ppm. The required chlorine injection rate could be cut in half by lowering the water pH from 7.5 to 6.5. |
Use this formula to establish the starting point for liquid sodium hypochlorite injection:
I liquid chlorine = (0.006 x P x Q)/m
I = gallons of liquid sodium hypochlorite injected per hour,
p = parts per million desired,
Q = system flow rate in gpm,
m = percentage chlorine in the source (usually 5.25% or 10%).
Chlorine injection example #2Determine the liquid chlorine injection rate given these conditions: 1) the chlorine source is a 10% sodium hypochlorite solution, 2) the system flow rate is 1000 gpm, and 3) the desired concentration is 5 ppm. Answer(0.006 x 5 ppm x 1000 gal/min)/10 = 3 gph |
Use this formula when injecting chlorine gas:
I gas chlorine = (0.012 x P x Q)
I = chlorine gas injection rate (lbs/day),
P = parts per million desired,
Q = system flow rate in gpm.
Chlorine injection example #3Determine the gas chlorine injection rate given these conditions: 1) the system flow rate is 1000 gpm, and 2) the desired concentration is 5 ppm. Answer(0.012 x 5 ppm x 1000 gal/min) = 60 lbs/day |
Take these precautions when injecting chlorine:
Copper sulfate is occasionally used to prevent bacterial growth in micro-irrigation systems due to the toxicity of copper.
Chlorine is more effective, less expensive, and causes fewer plant toxicity and aluminum corrosion problems than copper sulfate. However, it is now included in some commercial irrigation line cleaning mixtures together with citric acid to reduce slimy bacterial growth.
Copper sulfate is commonly used for pond treatment to suppress algae. Even at relatively high concentration (around 30 ppm), copper sulfate will not be completely effective because algal spores can exclude it.
Chelated copper acts as a bactericide similar to copper sulfate. Its advantage is that it is effective when injected at much lower rates (about 1 ppm).
It is usually not necessary to inject copper compounds for an entire irrigation cycle. Copper can be injected during the latter part of the cycle, which leaves an effective residue in the lines to prevent bacterial growth after the system is shut down.
| Carbonates in water | N-pHuric® 28/27 | N-pHuric® 15/49 | N-pHuric® 10/55 |
| PPM | OZ | OZ | OZ |
| 50 | 12 | 6 | 6 |
| 100 | 24 | 13 | 11 |
| 200 | 49 | 25 | 22 |
| 300 | 73 | 28 | 33 |
| 400 | 97 | 50 | 44 |
*CAUTION: Always add acid to water; do not add water to acid. Adding water to acid can cause a violent reaction, and may cause the acid to splash on the person pouring the water. Individuals working with acids should wear protective clothing and eyewear. Also, be sure that adequate safety devices are provided, including a shower and eyewash.
The most common alternative chemicals to chlorine and acids are scale inhibitors, which lessen scale formation by preventing precipitation reactions from occurring long enough for problem ions to clear the irrigation system. Inhibitors are usually synthetic polymer or polyphosphate mixtures. They are often combined with surfactants and penetrants to help break apart biological and crystalline solids attached to tubing walls and emitters. Water conditioners do not kill bacteria, but they alter the chemical environment so conditions for deposition or attachment are less favorable.
Scale inhibitors have been used for many years in municipal water treatment and cooling tower applications.
Scale inhibitors can be costly because they are usually proprietary materials.
There is uncertainty about inhibitor selection and injected concentration required
The most common synthetic scale inhibitors are polyphosphates:
There are major differences between polyphosphate compounds. Comparisons of one chemical with another are complicated and depend on water chemistry
Polyphosphates vary in their ability to trap and hold metal ions in solution. The effectiveness of a polyphosphate is determined by:
Typically, polyphosphates cannot sequester more than 1 to 2 ppm Fe in irrigation water, and the dosage required is 2 to 5 times the Fe concentration.
Polyphosphates usually lose their potency with storage times longer than about 4 weeks.
Phosphonates and polyelectrolytes have also been used in municipal water industries as scale inhibitors. They differ structurally from polyphosphates.
A polymaleic anhydride anionic terpolymer is currently sold to sequester iron and manganese.
Determine if a water conditioning treatment (injection of chlorine, acid, or a synthetic scale inhibitor) is working by monitoring the system or by installing scale-monitoring devices.
Keep track of system pressure:
Alternatively, keep track of system flow:
A scale-monitoring device is a clean, non-scaled surface like a glass slide or short section of new tubing that is spliced into an irrigation lateral line.
Install several devices across the irrigation system network, from laterals close to the pump to those at the far end of the system.
Irrigate with the system as normal, and periodically check the devices for new scale deposition.
When trying a new water treatment chemical, leave untreated at least one irrigation zone that draws from the same water source as the treated zones, and install scale monitoring devices in each.
After a 4 to 6 week trial period of irrigation in treated and untreated zones, examine the scale-monitoring devices to see if less scale was deposited in the zone where the water treatment chemical was used.
Skip to Top